The AHS Blog

The last piece of the jigsaw: a relatable story for researchers
This post was written by Su Fulwood
Research is, of course, interwoven through my work. One long-term project is about women and their roles in horology. This started when I was working with watchmaker Geoff Allnutt in his shop and workshops at 8 West Street, Midhurst. Geoff challenged me to go through lists of watch and clockmakers to note down all the women mentioned. After finding hundreds of women among the men, one name stood out: 'Mary Ann Lawrence, West Street, Midhurst'.
A quest began in earnest to find out where in the street Mary Ann had worked.

Years have passed and our research has covered many individual women, including, gradually, lots about Mary Ann. We discovered that she was actually from a well-known family of local watchmakers, the Wrapsons. We found that she took over her brother's premises in West Street following his death. We also discovered that she was close friends with fellow watchmaker, Joseph Charles Ketterer.
Ketterer, we knew with certainty, had run his business until 1914 from the premises Geoff now occupies.
We couldn’t say, however, whether Ketterer took over from Mary Ann or whether she had worked from another shop. It wasn’t uncommon in Midhurst for there to be two watchmakers in the same street at a similar time.
We did know Mary Ann retired around 1895, and that Ketterer was the executor of her will when she died. We just didn't have that last all-important piece that would tell us if she was part of the history of 8 West Street. It was frustrating.

Until now that is ... seven years later, when a chance conversation revealed the existence of a different advert for Joseph Charles Ketterer’s business.
When we saw it we couldn’t believe our eyes. There at the top, unexpectedly, was exactly the information we had been looking for. The words 'J. C. Ketterer (Late Lawrence)' were pure gold for us. It meant that Mary Ann Lawrence (who describes herself as 'Mistress Watchmaker' in the 1881 Census) was running her watchmaking business from the shop before Ketterer. This also meant that the same premises had belonged to her brother Charles Wrapson and, before him, their mother, Mary Wrapson.

Our research began by looking at watchmaking women across the country. But it has become very personal, as finally we were able to show that two of those women inhabited the exact spaces we work from today.
In the meantime, along with my research about women and horology, I get to work with other women today, as more are attracted back to watch and clockmaking.
The tools are important too
This post was written by Seth Kennedy
Many horological workshops contain old tools. It seems to go with the territory. There is something about using a hand tool that has already been around for a century or more. It brings questions, wondering whose hands have previously worked with this object over several generations and what timepieces were they working on?

With thanks to a fellow restorer, I recently became custodian of something a bit larger than a saw or a file. It’s a plain lathe by Pfiel most likely made in the late 19th century. Pfiel & Co were tool makers and retailers with a large shop and warehouse on St John Street in Clerkenwell. There are a few details about these lathes that make them distinguishable and so it is possible they were actually cast and machined in London to Pfiel specifications.
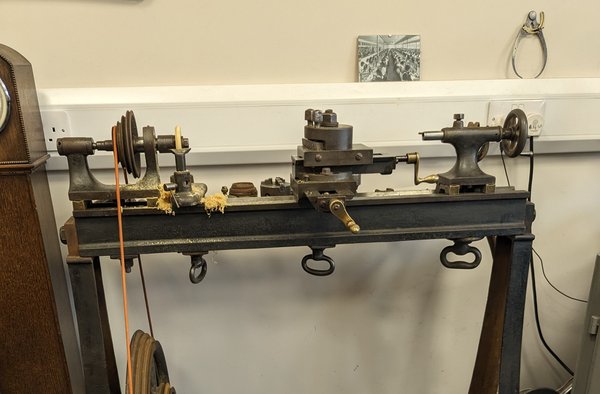
The centre height of the spindle is 3.5”, or at least it was, since on this example some home-made brass spacers have been added under the head and tailstock so that larger parts could be turned. These spacers fit the flat front and veed back surfaces of the lathe bed.
The treadle board itself is an incredible survivor, showing its wear of a century of use. Also remaining, though unusable, is a very old gut line that was previously used to connect flywheel to spindle.


What makes this lathe particularly special is that its history is known. It came from the workshop of Daniel Parkes, a fourth generation clockmaker/restorer who was active in Clerkenwell until retirement in 1989. He was a founder member of the AHS and some of his tools are on display in the museum of The Worshipful Company of Clockmakers. Parkes used only traditional equipment and methods in the restoration of fine English clocks of the 17th and 18th century golden age.

The lathe could have come from his family’s workshop on Spencer Street, which was destroyed by a V1 bomb in June 1944 but from which he salvaged some equipment. Or it might have already been in the workshops that Parkes then moved to on Brisset Street, where a partnership was formed with A. & H. Rowley, also a firm with long Clerkenwell roots.
Either way, someone in one of those businesses needed a lathe in around 1890 and so walked down the road to the Pfiel shop and bought this one. After Parkes’s retirement it went to Mike McCoy, then to Matthew Hopkinson and then recently to my workshop.
It has now had some cleaning, oiling, a hidden strengthening plate added to the treadle, a new leather belt fitted and a new shelf added as the original had gone missing.
Tools are meant to be used and so, after perhaps 30 years of slumber it will now occasionally be put into action again, for turning boxwood chucks used in watch case making and other light tasks.

Backwards and forwards
This post was written by James Nye
I am shocked to realise it is now a decade since I blogged about Enicar watches. As collectors know, Enicar derives from reversing the family name of the owners, Ariste and Emma Racine. Signing clocks and watches like this has a long tradition, with an interesting variety of explanations.

Back in the December 1993 issue of Antiquarian Horology, Sebastian Whitestone discussed some watches that bogusly claimed a London origin, but which in reality were from the Friedberg region and signed Lekceh, Legeips, Rengaw, Renpuarg and Ysorb. Reverse these and we have the less exotic Heckel, Spiegel, Wagner, Graupner and Brosy.
Mirrors reverse images, and tellingly, Spiegel also used the giveaway signature Miroir, London. The word ‘spiegel’ is German for mirror, and thus another pun.
But not all Friedberg makers reversed their names when they added a London location. Eckert and Schreiner signed (falsely) from London, but used their normal names.

Surveying the Ashmolean Museum's watches in the December 2000 issue of AH, David Thompson enlarged the geography, tying a watch signed ‘Renpuarg, London No. 4’ to Augsburg.
Alice Arnold Becker revisited backward signatures in AH in June 2014, taking the line that it was all marketing. The watchmakers perhaps falsified locations to secure the gravitas and caché of the London market – creative branding, familiar today.
Dutch horologist John Selders has also studied this phenomenon, publishing several pieces in Tijdschrift and summarising in 2015 with a list of 24 such reversed names, from the late seventeenth century to the turn of the nineteenth, and noting that some makers signed from Paris, not just London.
Strangely, precisely the same thing happened in Britain. English and Scottish makers of watches also reversed their names. An example is a watch by James Peddie of Stirling, signed on the back plate Jas Eiddep (see Clocks, April 2019). More prolific is Eardley Norton, whose signature Yeldrae Notron appears with surprising frequency. Usher & Cole’s apprentice John Wontner later signed objects as Rentnow. Cole signed as Eloc.

What on earth were all these people doing? There are several theories. Some assume distant makers wanted to capture borrowed prestige, and value, by signing from London, or Paris – an element of passing-off. A false signature disguises true authorship.Some suggest it was done to avoid taxes. Perhaps it limited redress to a real maker. Or perhaps it shows the maker’s sense of humour?
Does it all echo the original Rolex/Tudor division – an affordable product from the same stable, but differentiated?

No firm conclusions are possible, but some theories are surely too far-fetched. Tax officials won’t be fooled by mirror-writing. Signing a watch carries a maker’s chosen identity out into the world, and should be permanent, and memorable. A sense of whimsy could be involved, maybe even a sense of a brand that stands on its own, distinct from the name of the controlling mind involved.
In all, a subject that deserves more than a backwards glance... and if anyone has any other examples of reversed clock and watch signatures, please do tell me!
The watches of Samuel Pepys
This post was written by Peter de Clercq
The office of the AHS in Lovat Lane in the City of London is a stone’s throw away from The Monument, erected to commemorate the Great Fire of London of 1666.
The fire was famously reported in The Diary of Samuel Pepys (1633–1703), which ‘is unique, no less for Pepys’s self-revelation, than for its graphic picture of social life at the time of the Restoration’. This is a quote from the preface in my copy of the Library of Classics edition first published in 1825. Running to 640 pages, it yet contains scarcely half of the manuscript. It had also been ‘suitably edited’, which perhaps means that for example anything to do with his bodily functions or his amorous exploits had been removed.

A more complete edition appeared in 1893, and this forms the basis for a magnificent website which offers ‘the full text of his diary, along with several letters sent or received by Pepys, plus thousands of pages of further information about the people, places and things in his world’.
It is searchable, and if you want to find out what Pepys wrote about clocks and watches, you will find some two dozens entries.* Let’s follow one trail, all to do with watches:
Monday 17 April 1665: 'This day was left at my house a very neat silver watch, by one Briggs, a scrivener and sollicitor […].'
Friday 12 May 1665: 'To the ’Change [the Royal Exchange] and thence to my watchmaker, where he has put it [i.e. the watch] in order, and a good and brave piece it is, and he tells me worth 14l. which is a greater present than I valued it.'
Saturday 13 May 1665: 'To the ’Change after office, and received my watch from the watchmaker, and a very fine [one] it is, given me by Briggs, the Scrivener. […] But, Lord! to see how much of my old folly and childishnesse hangs upon me still that I cannot forbear carrying my watch in my hand in the coach all this afternoon, and seeing what o’clock it is one hundred times; and am apt to think with myself, how could I be so long without one; though I remember since, I had one, and found it a trouble, and resolved to carry one no more about me while I lived.'
Wednesday 13 September 1665: 'Up, and walked to Greenwich, taking pleasure to walk with my minute watch in my hand, by which I am come now to see the distances of my way from Woolwich to Greenwich, and do find myself to come within two minutes constantly to the same place at the end of each quarter of an houre.'
Friday 22 December 1665: 'I to my Lord Bruncker’s, and there spent the evening by my desire in seeing his Lordship open to pieces and make up again his watch, thereby being taught what I never knew before; and it is a thing very well worth my having seen, and am mightily pleased and satisfied with it.'
Saturday 22 September 1666: 'My Lord Brunck […] now give me a watch, a plain one, in the roome of [instead of] my former watch with many motions which I did give him. If it goes well, I care not for the difference in worth, though believe there is above 5l.'
All this is very interesting, but it leaves questions unanswered.
Why was Pepys given a watch 'by one Briggs, a scrivener and sollicitor', about whom we hear no more in the diary, and who is not mentioned in Claire Tomalin’s excellent biography published in 2002? Did he want to curry favour with Pepys, perhaps hoping to benefit somehow?

We are not told the name of the man who had serviced his watch, nor do we have details of the watch except that it was worth £14 and came 'with many motions', which we take to mean that it not just showed the time but also had additional functions that could make a watch such an attractive possession.

But why, we may ask, had Pepys earlier in his life been so frustrated with a watch? Had it malfunctioned?
His wife had her own watch (Saturday 9 February 1666/67: 'This noon come my wife’s watchmaker, and received 12l. of me for her watch') but it led to irritation (Wednesday 3 April 1667: ‘and to bed, vexed … that my wife’s watch proves so bad as it do').
Or had it been the anxiety that a watch could draw unwanted attention, like we hear these days of brutal daylight robberies of expensive watches? On Wednesday 1 January 1667/68, Pepys ‘took coach and home about 9 or 10 at night, where not finding my wife come home, I took the same coach again, and leaving my watch behind me for fear of robbing, I did go back.’
If he was so taken with his fancy watch that he could not stop consulting it when he set out with it in his coach, why did Pepys later give it away to William Brouncker (c. 1620–1684), and accepted a simpler and less valuable one in return?
Lord Brouncker was from 1662 to 1677 the first president of the Royal Society, an office to which Pepys himself was to be appointed in 1684, long after he had stopped writing his diary.
Brouncker features prominently in Pepys’s diary; they were neighbours and on friendly terms. So perhaps Pepys gave him his fine watch simply from the goodness of his heart, and that is a nice note to end on.
* Searching on ‘clock’, one must weed out hundreds of 'o’clocks', and ‘watch’ is of course often found to mean ‘look at’, but with some patience I found 23 pertinent entries which I shall be happy to share with anyone interested.
Ringing in the New Year
This post was written by Andrew Strangeway
As a clockmaker, or practising horologist, there is one particular moment while waiting for a clock to strike when time seems to slow down.This moment is more daunting when the streets outside are lined with 100,000 people and millions more are watching live on television, and streaming, waiting to hear the clock chime.
On December 31st it all comes down to a single moment, when the nation watches and waits for Big Ben to ring out and signal the start of the new year. Behind the scenes, however, months of preparation go into ensuring that this first hammer-blow falls precisely on time. Besides the installation of additional broadcast equipment, regular servicing, and thrice weekly winding there is also timekeeping: ensuring, literally, that the clock stays on time.
In the months leading up to the end of 2023, the timekeeping of the Great Clock was a core focus. After the break of six years during the servicing of the clock and tower, the twice-daily live chimes finally returned to BBC radio airwaves on November 6th. This New Year marked the 100th anniversary since the bells were first broadcast live for New Year’s Eve. It was a matter of pride that the clock should perform well and strike on time.
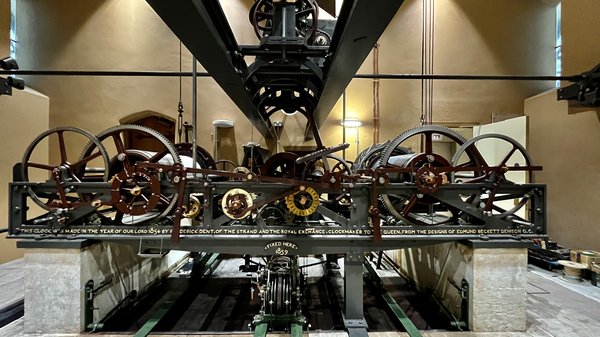
When it was first installed, the Great Clock was, by some margin, one of the most accurate public clocks in the world. The original specification for the clock set down in 1846 by George Airy, the Astronomer Royal, required that ‘the striking machinery be so arranged that the first blow for each hour shall be accurate to a second of time’. As clockmakers our job is to ensure that the clock remains true to this original purpose. To our advantage, we have highly accurate timekeepers to compare the Great Clock against and, armed with this information, we can regulate the pendulum carefully and actually comfortably exceed the original target for accuracy.

Since starting at the Palace of Westminster in August 2023, I have implemented the use of a new custom-made timer to increase the accuracy of the timing of the first hammer-blow of the hour and an improved regimen of regulating the rate of the clock’s pendulum. The core mechanics of regulation remains as ever: the placing or removing of weights, in the form of old currency, on a small platform partway down the length of the pendulum rod. Famously, one pre-decimal penny added speeds up the clock by about 2/5th of a second over 24 hours. These are a little too weighty and the adjustment too coarse if one is aiming for a finer degree of accuracy. I have begun using ha’pennies and farthings for regulation, kindly lent for the purpose by my colleague in the clock team, Ian Westworth. These speed the clock up by about a quarter of a second a day and a tenth of a second a day respectively.

In the final days running up to New Year’s Eve I was at the top of the tower, on the hour, throughout the day to take a timing reading from the strike and to make small adjustments to bring the clock precisely to time. As luck would have it, on the evening of the 30th we experienced heavy winds, which buffeted the tower and were enough to throw the clock out by 0.2 seconds by the morning of New Year’s Eve. I was able to correct for this by the evening. Midnight came and Big Ben struck, and the view of the fireworks from the top of the Elizabeth Tower was astounding. It was awesome to see the fireworks perfectly synchronised with the strike of the bell.

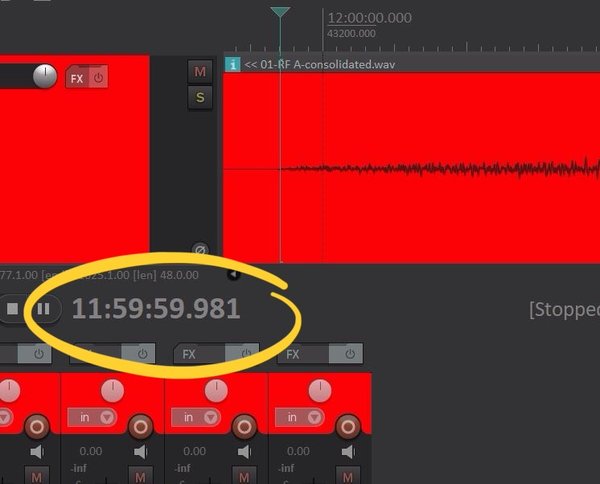
My timekeeping efforts were rewarded when we measured the timing of the strike from the sound recording compared with a GPS clock – these results were generously provided by Mark Powell of DeltaLive. The result was that Big Ben struck midnight at exactly 23:59:59.981, within less than a fiftieth of a second of midnight. That’s less than a fifth of the time it takes to blink your eyes. I must confess to being rather pleased with that!

Horology in Oxford museums
This post was written by Peter de Clercq
Long ago I reported on clocks in Berlin museums and more recently on clocks in Lisbon. Let me now direct you to three museums in Oxford, home of the oldest university in the English-speaking world and the world's second-oldest university in continuous operation.
The Ashmolean Museum is the University of Oxford’s museum of art and archaeology, founded in 1683. Its world-famous collections range from Egyptian mummies to contemporary art. It has a large collection of watches, which came to the museum largely as a result of three major bequests. Together these make the Ashmolean collection of watches one of the most important outside London.
You find them on display on the second floor in Gallery 55, and photos with descriptions of some 70 of them on the museum’s website. In 2008, David Thompson, then curator of Horology at the British Museum and currently a Vice-President of the AHS, presented some 30 highlights in a 96-page book which is on sale at the museum’s shop for a mere £5.

The Ashmolean Museum started in a building on Broad Street, and, after it moved to its new premises in the nineteenth century, the ‘Old Ashmolean’ building was used as office space for the Oxford English Dictionary. Since 1924, it has housed another University museum, the Museum of the History of Science, or the History of Science Museum as it was recently renamed.
In the entrance gallery stands a longcase clock made by Ahasuerus Fromanteel in around 1660, acquired by the museum in the 1930s and restored in 2008 with financial support from the AHS. This was an occasion for the then journal editor Jeff Darken to present the clock in enormous detail in a 22-page ‘Picture Gallery’ article in the December 2008 and March 2009 journals, of which two sample pages are seen here.
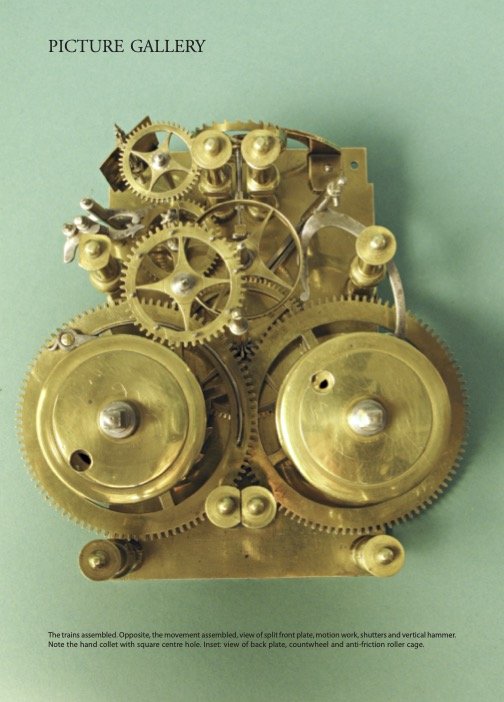
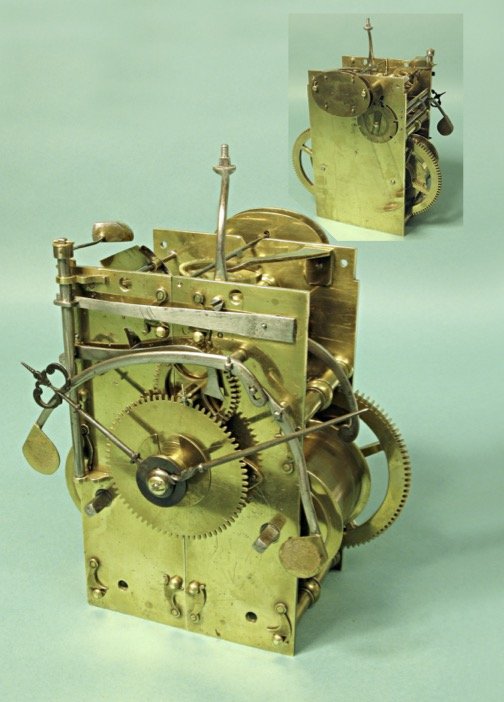
In the basement, more clocks and watches are on display. Two unusual objects in the collection that I particularly like are an oven and an icebox used to check the performance of chronometers at high and low temperatures. They had been used by the London-based watch and clockmakers Daniel Desbois and Sons.


AHS founder member Michael Hurst (1924–2017) has told me that he and his brother Lawrance (1934–2018) collected the oven and icebox from the Desbois premises in Brownlow Street, Holborn, in about 1955 and stored them on behalf of the AHS in their family home in Hendon. They were later stored by the British Museum and subsequently donated by the AHS to the Oxford museum in 1972.
They are currently in the museum store, but were brought out for an exhibition ‘Time Machines’ in 2011–12, which offered a distinctly inspired ‘new way of seeing the Museum’s exceptional timepieces’. They first featured in my blog ‘Chronometers in hell’, posted on July 26, 2015.
A third place where we find some time-related objects is the Pitt Rivers Museum. It was founded in 1884 by Augustus Pitt Rivers (1827–1900), an English officer in the British Army, ethnologist, and archaeologist, who donated his private collection to the University of Oxford. The museum is entered through the Oxford University Museum of Natural History, and through that inner door you step into an amazing Aladdin’s cave brimming with display cases and exhibits.

An unusual and distinct feature of this museum is that the collection is arranged typologically, according to how the objects were used, rather than according to their age or origin. It makes for unexpected juxtapositions. This also applies to the case on the first floor gallery which contains seventeen objects on the theme of time.

To highlight just four, there is an intriguing double-bowl clay pipe with bamboo stem from India, Nagaland, Kaly-Kengu. As the label informs us, ‘Each bowl is smoked separately to measure the time spent working in the fields.´ Top left is a copper water-clock from Sri Lanka, with caption, ‘Such clocks have a tiny hole in the base so that when they are set on the surface of a bowl of water they float for a known period of time. This example is said to sink after 11 minutes.’ From Tyrol, Austria comes the ‘Pewter clock-lamp calibrated to show the time from 9pm to 7am. As the oil in the lamp burnt the level fell, and the time was measured against the markings on the metal stand.’ And from Wimpole Street, London, comes the ‘Sand-glass used by dentists to time the mixing of fillings so they do not de-sterilise their hands by handling a watch. Donated by J. P. Mills 1939’.
Light among the lanterns
This post was written by James Nye
I used to walk regularly from Cannon Street tube station to the Clockmakers' Company office in Throgmorton Avenue, and on the way would walk down the west flank of the Bank of England, turn right and cross over to slip down Tokenhouse Yard. One day, passing St Margaret’s Lothbury, I stopped, and looked around. It struck me forcibly that Lothbury was really short, yet I knew that a fair number of lantern clocks were signed from addresses on the street. I was intrigued. Was this like restaurants crowding together and all benefiting from increased business?
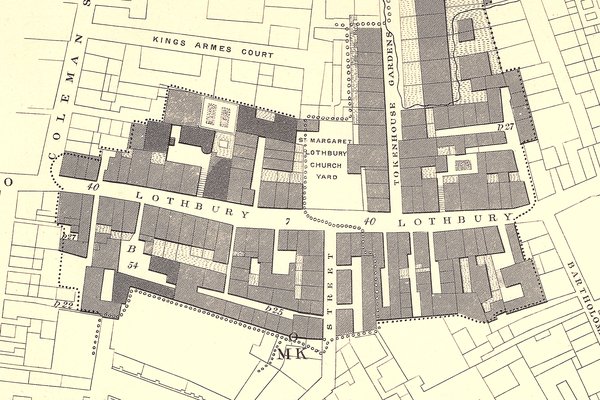
This was back in 2018, and I conceived the idea of a deep dive into the street’s pre-Fire history. It was known for its founders, and for their ‘turning and scrating […] making a loathsome noise’. Was it such a bad place? Who else lived there, and what did they do? I recruited a research assistant, Caitlin Doherty, and together we planned trips to archives and libraries, following up hunches and leads developed together.
This all resulted in a lecture we jointly gave in November 2019, but I had to jettison more than half my material to squeeze it into a decent length. Then Covid struck and the larger work languished, until mid-2022 when I dusted it down and gave it a read. I was pleasantly reminded of much, but also conscious that a lot more could be done, so I strapped on the tanks and went overboard again.
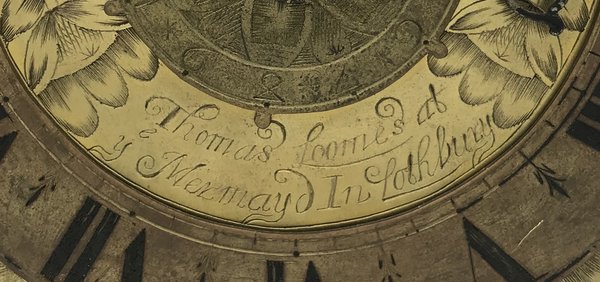
I was still frustrated that on the first attempt I had failed to pinpoint the location of the Mermaid, the best-known Lothbury address, home to Selwood, Loomes and others, and from which the first pendulum clocks were sold in London. A punch-the-air moment occurred earlier this year when I finally found archival documents that revealed its whereabouts.
The project eventually grew to a book-length treatment and I am deeply grateful to the Society for backing its publication. It has recently been rolling through the presses. You can see pages being prepared for sewing here and the cover being embossed here.

Make no mistake, this is not a technical book about lantern clocks. But it is a forensic account of the small community in which one group of remarkable makers lived. It reveals who their neighbours were and the challenges they all faced, whether against the backdrop of the Civil Wars or the poisonous smoky atmosphere. It discusses the trades in which the merchants on the street were engaged, but reveals some of the poverty close at hand. I hope that in many ways it should throw some more light around the early life of many lantern clocks. And you can order it here!
Time's up!
This was post was written by Jonathan Betts
Have you ever had to endure a relentlessly boring after-dinner speech?
In these less-formal days there are fewer opportunities, but in the ‘good old days’ in the late-nineteenth and early-twentieth century, when many of the elite of British society belonged to exclusive dining clubs, speeches could go on seemingly forever!
The Sette of Odd Volumes
The 'Sette of Odd Volumes', one of the more eccentric of such dining clubs, was founded in 1878 by the celebrated antiquarian bookseller, Bernard Quaritch (1819-1899). Over the following decades it was distinguished by having as members and guests many great achievers in the literary, scientific and academic worlds.
Horologically, our very own Rupert T. Gould (1890–1948) joined in 1920, and George C. Williamson (1858–1942), the antiquarian who published horology’s largest book, the catalogue of the Pierpont Morgan collection of watches, was also a member.
Members of the club were ‘brothers’ and adopted titles appropriate to their professions. Gould was ‘Hydrographer’ and Williamson was ‘Horologer’. The enforced, tongue-in-cheek jollity of the dinners required the exclusive use of their titles.

Oscar Wilde occasionally attended these Sette dinners as a guest. In later years, Wilde’s second son, Vyvyan Holland, was a Sette member, 'Idler', and was also a good friend of Gould's. Another great friend was the biologist and microscopist Edward Heron-Allen (1861–1943), ‘Necromancer’, who had joined the Sette back in 1883.

Each year, the Sette elected a new President, who sat in a grand throne-like chair when in office, and a Vice President, who would be President the following year. In 1891 Heron-Allen was about to be elected Vice President, and as a gift to the Sette (and as something of an aggrandisement to his new office!) he commissioned and presented a Vice President’s chair to accompany the President’s.
Those speeches
The Sette's activities were regulated according to a set of Rules, some of which were positively Pythonesque. For example, rule XVI was that 'There shall be no Rule XVI', and Rule XVIII insisted that 'No Odd Volume shall talk unasked on any subject he understands'.
One of the more sensible rules, however (No. XX), stated that 'No O.V.’s speech shall last longer than three minutes; if however, the inspired O.V. has any more to say, he may proceed until his voice is drowned in the general applause'.
Finding that this rule was constantly being disobeyed, and that evidently the ‘general applause’ was insufficient, in December 1891 Heron-Allen also commissioned and presented to the Sette an elaborate speaker’s bell, to ensure they stuck to time.


The bell
Made by the well-known Victorian electrical engineers, Woodhouse & Rawson, the bell is of exquisite quality throughout. Constructed in a mahogany case containing the electro-mechanical timer, the large bell, mounted on the top, has a slender rod running vertically through it, which had (now missing) a ball on top.
In operation, reminiscent of the way a time ball runs, the timer is set going and the ball (which was probably an ivory billiard ball originally) is seen to slowly climb up from the bell. The ascent is controlled by a clockwork worm-and-fly governor, and at the point at which the three minutes has elapsed, the ball, now about 2.5 inches above the bell, suddenly drops back down to the bell, which then begins to ring very loudly, an electrical make-and-break hammer operating on the inside.

For the speaker, having a miniature time ball inexorably rising in front of him while he was talking must have been quite off-putting and, needless to say, the timing device was not popular. One can only imagine what Oscar Wilde would have thought (and said!) if the thing was set going as he began giving an after-dinner speech!
In fact, a certain flexibility was built into the controls of the bell, and the operator (presumably the Master of Ceremonies) was able to pause the clock, or even ring the bell in advance, if deemed necessary!
But its presence as a gift would soon be quietly but firmly removed anyway, and this fascinating and beautiful object, and the Vice President’s chair, were not seen again. This was owing to an incident which left Edward Heron-Allen unpopular among the elders of the Sette and which caused him to resign from the Sette a year or so later.
The incident, which is described in an excellent book by John Mahoney (The Richard Le Gallienne versus Edward Heron-Allen ‘Incident’ at The Sette of Odd Volumes, privately published, Tallahassee, 2023), concerned the proposal for membership of the Sette of the author and poet Richard Le Gallienne (1866-1947).
Le Gallienne had had a brief affair with Oscar Wilde, and it seems that at the time he was being proposed for the Sette, Heron-Allen had made a remark in confidence at a private dinner party about Le Gallienne’s sexuality. Unfortunately, this remark was not treated in confidence, and Le Gallienne got to hear of it. This would, given the illegality of homosexuality at the time, be a very serious matter for him.
The scandal which ensued polarised views within the Sette, causing Le Gallienne’s election to be dropped temporarily and Heron-Allen to later resign. The chair and timer were thus quietly put away, though the chair does in fact remain in the Sette’s possession; the timer escaped at some stage and only resurfaced a few years ago.
For Heron-Allen the story ended happily, however. He would re-join the Sette thirty years later, and he enjoyed many a dinner with Gould, Holland and his other friends, no doubt having to put up with the long speeches after all.

A varied horological life
This post was written by Su Fulwood
'I have to ask you, is this your actual job?' This was put to me last week while I was winding one of the clocks in the collection at Goodwood House, the home of The Duke and Duchess of Richmond in West Sussex. Well, yes it is – one of them anyway, I do a few things.
I’m a freelance collections advisor, researcher and curator, so I work with all things from costume to archaeology, but more than half of my projects are horology related. Possibly this is because clocks, as something with a beating heart metaphorically, evoke memories and stories as well as encompassing art, science, technology and history, so as an example of material culture they are multifaceted and valued.
More than anything else, clocks are firmly connected to people, their knowledge and emotions. Working with them enables me to meet and work with excellent people, to travel, and it defines the places I visit.

I’ve recently returned from a job in York that enabled me to explore the coast from Staithes to Scarborough (I suspect turret clock specialists can relate to this).
This year began with a project working with the fabulous Cumbria Clock Company and their newer recruits. I can't express how privileged I feel to work with both experienced clockmakers and the next generation of horologists.
Back at Goodwood, I have an annual presence at The Festival of Speed and The Revival exposing me to all manner of wonderful cars, their clocks and watches.

My research on women working in horology with Geoff Allnutt also leads to fantastic places. The horology community is unlike any other and this introduction is also a thank you to all at the Antiquarian Horological Society who have supported me with my work. More to come!
Two watches in literature
This post was written by Peter de Clercq
By way of summer entertainment, here are two watches, both regarded with incomprehension but for very different reasons.
In his famous book Travels into several remote nations of the world by Lemuel Gulliver, published in 1727, Jonathan Swift relates how, during his first voyage of 1699, Gulliver is washed ashore after a shipwreck and finds himself a prisoner of a race of tiny people, less than 6 inches (15 cm) tall, who are inhabitants of the island country of Lilliput.
At the Emperor’s instruction, two officers inspect the contents of his pockets and draw up an inventory. Everything they find – a hairbrush, a snuff box, knives for shaving and eating – is a mystery to them, and the same goes for what they find next.

‘Out of the right fob hung a great silver chain, with a wonderful kind of engine at the bottom . . . which appeared to be a globe, half silver, and half of some transparent metal; for, on the transparent side, we saw certain strange figures circular drawn, and thought we could touch them, till we found our fingers stopped by that lucid substance.
'He put his engine to our ears, which made an incessant noise like that of a water-mill; and we conjecture it is either some unknown animal, or the god that he worships; but we are more inclined to the latter opinion, because he assured us (if we understood him right, for he expressed himself very imperfectly) that he seldom did anything without consulting it. He called it his oracle, and said it pointed out the time for every action of his life.'

In 1894, Macmillan published a luxury edition of Travels ..., and commissioned the English painter, line artist and book illustrator Charles Edmund Brock (1870–1938) to prepare one hundred illustrations, including the watch scene.

Heinrich Christian Wilhelm Busch (1832–1908) was a German humorist, poet, illustrator, and painter. His wildly innovative illustrated tales have remained in print ever since they first appeared.
Probably the most famous one is Max and Moritz in which two boys are terribly punished for a series of pranks – many of Busch’s stories are not for the faint-hearted! I still treasure the edition I was given as a child, containing a selection for the young of the best-loved tales, all coloured-in (the original published editions were in black and white).
In the story Der Schreihals (The Bawler), dated 1869, we find the second watch. Ever since his mother has bundled him in, the baby boy has been screaming, nothing can make him stop, not even the father who tries to amuse him in various ways, including in the image captioned ‘Hear, little Willy, listen to the tick-tack watch / But Willy simply cries even louder’.
The ordeal only ends when an aunt comes to visit, and ‘Full of wisdom, she opens the bundle / There we have it! That was the cause!’
In the final image, ‘Willy, relieved of the pain / Laughs loudly for pure happiness.’



Clock confusion – think about it!
This post was written by John Laycock
Why do the numbers of 1 to 12 on a clock dial have to be multiplied by 5 to allow one to work out the time?
If there are 24 hours in a day then the daily time continuum is 0 to 24. Why then do mechanical clocks split this continuum into two 12 hour segments? If the continuum has to be split surely it would make more sense to split between day and night with the daylight spanning 6am to 6pm and then night from our 6pm to 6am!
Why is noon 12pm? It is neither am nor pm - simply because we have chosen to reset the continuum at 12! Is midnight 12am? Some digital clocks will show the 60 minutes after midnight as 12.xx whilst others will show the time as 00.xx!
Several attempts have been made to design an unambiguous dial using a 24 hour clock. The modern dial below is a good example.

The most important element is the hour (long hand) with the minutes as secondary (short hand). The dial is numbered with the hours from 0 to 23, as time will be read as hour plus minute and thus 24 will never be appropriate. The shorter hand points to an inner annulus numbered 0 to 55 in steps of 5 to avoid the necessity to perform the mental x5 operation to calculate the minutes or seconds.
Why has this relatively simple and unambiguous display of time not been universally adopted? We still do not think 24 hours we think morning and afternoon.
What about the time warp that occurs when reading minutes? With the minute hand in the right half of the clock face it is usual in the English language to interpret time as 'past the hour' but when the hand moves to the left half of the clock face we usually switch dimension to 'to the next hour' e.g. twenty past six, twenty to seven.
Are we using the clock to display past time or future time? Past time is historical and cannot be used again, future time is at our disposal and should be used to best advantage. Thus the clock face should logically portray future time and not past time.
Taking into account the discussion above making the case for the 24 hour dial, should the numbering system be completely reversed. The dial should display the hours descending clockwise from 23 to 0 and similarly the minutes descending clockwise from 55 to 0 in steps of 5.
Will it happen – NO. We have two much time invested in the past!
The evils of modern living
This post was written by James Nye
If you have not seen Terry Gilliam’s Time Bandits, I strongly recommend it. Without giving too much away, there are some magical (hilarious) scenes when Evil is incarcerated in the Fortress of Ultimate Darkness, planning the overthrow of the Supreme Being.
He believes he will be free, ‘Because I have understanding’. A flunkey asks, ‘Understanding of what, Master?’ Evil replies, ‘Of digital watches… and soon I shall have… understanding of video-cassette recorders, and car telephones, and when I have understanding of them… I shall have understanding of computers. And when I have understanding of computers, I shall be the Supreme Being.’
It is a 1981 film. Demanding explanations of all that is new in the world, Evil asks about fast breeder reactors. Receiving an explanation, he stares into the void. After a pause, he says, ‘Show me… Show me… Subscriber Trunk Dialling.’
For me, this was always the laugh-out-loud moment, that Evil could be so fascinated by STD.
The technology had been rolled out over the previous two decades, being completed in 1979. For a long time, telephone callers had been used to fixed periods of call charging, in units costing two pence each, and would hear warning pips as each period was near to ending. STD would do away with this, and two pence would now buy different quantities on a zone and time basis.
Smiths Industries anticipated a need for callers who might need reminding of the cost of their calls, and hence the Telephone Timer.
A turn clockwise of the central knob winds what is essentially an alarm-clock mechanism. The lever at the top starts and stops the clock. Once started, the dial visible through the top segment rotates anti-clockwise, and the reticle line shows call cost, based on one of four different rates.

Each two minutes, a bell is struck once, to jolt the caller back to consciousness of the pennies being frittered away in conversation.

Introduced in 1961, it was widely publicised, even meriting a full two-page review from the legendary T. R. Robinson.

Prescient as always, some of the evils Gilliam identified remain with us, while others have long since disappeared. The Smiths Telephone Timer falls very much in the latter category.

The National Physical Laboratory: paving the way for trusted time and frequency across the UK
This post was written by Leon Lobo
Time is the invisible utility underpinning capability across critical national infrastructure (CNI), as the basis for global navigation satellite systems (GNSS), synchronisation of 5G mobile telephone networks and the energy grid and timestamp traceability critical for trading platforms in financial services.
The world we live in is increasingly digital, with the last few years only accelerating this. We are seeing advances in communication and connectivity and society’s reliance on reliable positional, navigational, and timing (PNT) data is growing.
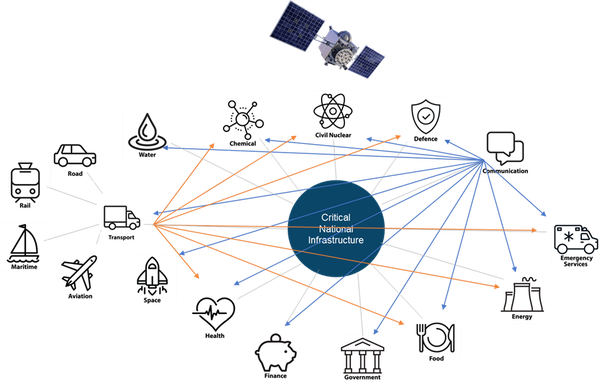
Many aspects of UK industry and society require increasingly precise time, whether it’s at home on our devices, the telecoms networks, energy, broadcast or in the finance industry where the time margins used are unfeasibly fine: one second for voice trading, one millisecond for electronic trading, and just 100 microseconds for high-frequency trading – all traceable to Coordinated Universal Time (UTC), the global time scale.
The systems, which rely on PNT signals from GNSS, are robust in themselves and have procedures in place to deal with any GNSS based system faults. However, disruptive interference can occur unintentionally and deliberately, with malicious interference a growing possibility. Potential interferences include jamming GNSS receivers, rebroadcasting a GNSS signal intentionally or accidentally, or spoofing GNSS signals to create a controllable misreporting of position.
An alternative timekeeping source is crucial to help prevent any serious impact to all of the above if something were to occur to the signals received via GNSS. They will also future proof society as we develop smart cities, autonomous vehicles and communications.
To mitigate these issues and vulnerabilities, the National Physical Laboratory (NPL), through its National Timing Centre (NTC) programme, is developing an alternative, potentially primary solution for future timing. The team and I are striving to ensure that the UK digital infrastructure is supported by a GNSS-independent sovereign capability for time moving forward. I am keen that we become more aware of our dependency on GNSS and start to consider how we do things differently. Dependence by default on this space asset is no longer tenable without due consideration for resilience of operations.

The NTC programme will provide the resilience needed to protect critical national infrastructure, keeping essential services running and ensuring trust in new technologies. It will enable the UK to move away from reliance on GNSS, like GPS, for time, and leverage a range of time and frequency distribution technologies including fibre, communication satellites, and terrestrial broadcasts, alongside GNSS.
NPL’s NTC programme aims to deliver a resilient UK national time infrastructure through the building and linking of a new atomic clock network distributed geographically in secure locations. It will also provide innovation opportunities for UK companies through funding projects in partnership with Innovate UK based on a successful NPL and Innovate UK partnership model. Finally, the programme will respond to the specialist skills shortage in time and synchronisation solutions through specialist, apprentice and post graduate training opportunities.
Improved resilience will help to strengthen our society through faster, more secure internet, robust energy supplies and reliable health and emergency services. As a community that understands timing and timekeeping, I am keen to have you engaged, raising awareness of why time is important to our daily lives and what we need to consider to ensure we have resilient time for the future.
Dr Leon Lobo is Head of the National Timing Centre at the National Physical Laborartory, whose work focuses on developing and delivering the UK's national timing strategy. Having worked at NPL since 2011, Dr Lobo has led the team managing the UK’s time scale and supported on the development of NPLTime®
The perils of poor lubrication and a potential solution
This post was written by JC Li
We all know the perils of poor lubrication: creeping oil, deterioration, wear… the list goes on. Good lubrication is paramount to the correct functioning and prevention of wear for horological mechanisms. Although modern synthetic oils have come on in leaps and bounds from the old-school vegetable oils and refined whale blubber, it can still prove challenging to ensure that they don’t ‘creep’ away from friction points to other areas. Where there is friction without oil there is wear, which can impede functionality and result in irreversible loss of material.


One solution used by some watchmakers is to apply a thin oleophobic (oil-repelling) coating known as epilame which helps retain oil at critical points, particularly in the escapement. However, it does not appear to be used as commonly by clock makers and repairers, typically oiling their escapements in the traditional way with no surface coating. As The Practical Lubrication of Watches and Clocks by the British Horological Institute (2008, p.15) states:
'Surface treatment to prevent spread of oils is not commonly used in clocks but can have similar advantages to its use in watches.'
As an MA Horology student at West Dean College, I’m hoping to establish through my thesis whether the use of modern epilame products such as Moebius Fixodrop or Episurf-Neo could be a conservation-grade solution to oil creep on pendulum escapements.
As such, it would be of great help to me if readers could respond to my two-minute survey about this topic, both in order for me to gauge current practices and also receive some feedback on this research. I equally welcome any pointers in this area of study or any relevant anecdotes of personal experience about oiling escapements, epilame, or any of the other issues referred to. I can also be contacted using my West Dean email address: s21jl@westdean.ac.uk. Thank you.
An animation of Thomas Mudge’s constant-force escapement
This post was written by Eliott Colinge
Visual representation has always been a core means to learn about horology and explore its principles.
The literature provides countless invaluable schematics about escapements, repeating mechanisms and patents of all sorts. Less can be said about video representations. If an image is worth 1000 words, what can be said about a video?
The recent popularisation of 3D animation in the film and gaming industry has gathered a wide community of brilliant individuals giving their improvements to the field at an increasing pace.
It is hardly possible to have a conversation about 3D animation without hearing the word 'Blender'. Being open source, the software has attracted students, researchers, professionals and amateurs around the world and is increasingly becoming a standard in the industry. Blender is such an incredible playground and I am always in admiration of what gets produced by the community.
As a trained conservator of horological artifacts, I founded the animation studio 'Vecthor' to provide complementary 'non-interventive' solutions to help education around collections.
Vecthor has made available a series of short animations of escapements based on drawings and a YouTube video about Pierre Leroy’s marine watch (available in English and French).
Here is an example through an animation of Thomas Mudge’s constant-force escapement used in his marine chronometers during the 1770s. The impulse energy is stored in the coiled springs (constant-force) and transmitted to the balance during its oscillatory arc.
Other escapement animations can be found on Vecthor's instagram: @vecthor_animation
A sad commemoration
This post was written by Jonathan Betts
A couple of weeks ago, I attended Bob Frishman's NAWCC conference on 'Great Horological Collectors' at the Horological Society of New York's home in New York City – a wonderfully successful and interesting event. By good fortune the date of the conference came just before a short holiday we had planned in New York, with passage home on the Queen Mary 2 – a delightful way to cross the pond.
When planning the trip I realised that, by a rather extraordinary coincidence, during the transatlantic crossing there would occur the 80th anniversary of a family tragedy, played out in those very waters.
My Uncle Pat – my mother's brother – was serving on a merchant troop ship, the MV Abosso. On the evening of 29 October 1942, when the ship was travelling alone in the North Atlantic, it was torpedoed by a U-boat and sunk. 362 of the 393 passengers and crew lost their lives, including Uncle Pat, who was just 23 and had married only a few weeks before.
Just one lifeboat survived with 31 souls to tell the tale, some of them Navy officers who were aware of the detail of what happened.
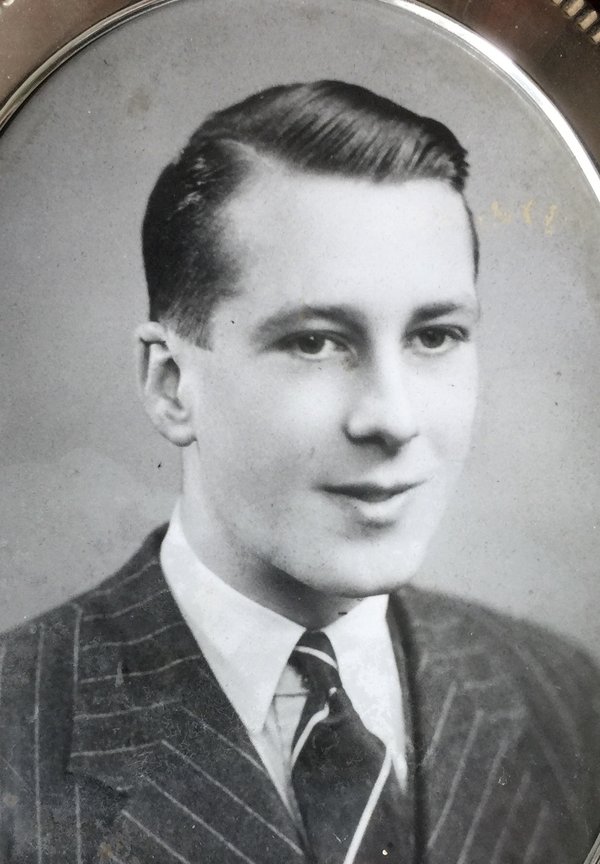
The reason for telling this sad tale in an AHS blog is that there were two critical horological elements to the tragedy.

About 6 p.m. that evening, the master of Abosso, Captain Reginald Tate, became aware the ship was being tailed by a U-boat – U575, commanded by Kapitan-Leutnant Gunter Heydemann (1914–1986), as it turned out. The ship's crew was able to detect the submarine using ASDIC (Anti-Submarine Detection Investigation Committee) echo-sounding to determine the sub's bearing and distance, the latter by using an ASDIC chronograph.

The watch has a high frequency sweep-seconds hand on a dial recording up to six seconds, the time between transmission of the sound pulse and the echo determining the distance of the sub from the ship.
Having established precisely where the sub was, the ship's master had to decide what to do.
An option was to go full steam ahead and try to out-run the submarine, which would normally be possible – a submerged submarine makes much slower progress, though at a maximum speed of 14.5 knots, the Abosso was not a particularly fast ship.
The alternative was to adopt a 'random' zig-zag course so the U-boat could not predict the ship's position accurately enough to make it worth firing torpedoes at it, though zig-zagging naturally slowed down the progress of the ship considerably. The practice did generally work well, if maintained long enough, as U-boats had limited quantities of fuel and eventually had to head off for a rendezvous for refuelling.

Deciding not to try and out-run, but to zig-zag, the Abosso brought into action its zig-zag clock, a part of the navigational equipment on the bridge of WW2 merchant ships at the time.
A fairly standard ship's bulkhead clock is adapted to have an electrical contact at the tip of the minute hand, and round the periphery of the minute track on the dial is a metal ring with a number of moveable electrical contacts.
Each time the minute hand touches a contact it closes a circuit and a bell rings in the wheelhouse informing the crew to change course one way or the other.
If in convoy with other ships it is of course vital that all the ships adopt precisely the same agreed pattern of zig-zags, and when the instruction to zig-zag is given, the whole convoy would be signalled a secret code for the pattern to be used. The contacts on all the ships’ clocks would then be adjusted accordingly and zig-zagging would begin at a specific time, thus foiling the U-boats' attempts to align torpedoes with a target ship.
The log of U575 has survived, so we can also follow what happened from the U-boat’s perspective. Heydemann, a much decorated Commander in the ‘Brandenburg’ wolfpack in the North Atlantic, sighted the Abosso about 6 p.m. that evening. He was running low on fuel but decided to try and attack. Realising he was being traced, he went deep, out of reach of the ASDIC, but followed from a distance until he was nearly out of fuel. At that point, thinking they had lost the sub, Abosso’s master made the fatal decision to stop zig-zagging. Realising this, Heydemann risked running out of fuel and moved closer to the ship to attack.
Reading the U-boat's log today, and comparing with the U-Boat Commander’s Handbook (available as a translated reprint as E. J. Coates (translator), The U-Boat Commander’s Handbook, Thomas Publications, Gettysburg, 1989) one discovers this was a textbook operation.
At 22.13hrs, Berlin time, one of the middle torpedos of four directed at the Abosso in a fan pattern struck the ship virtually amidships. The U-boat surfaced to inspect the target and Heydemann recorded seeing lifeboats being launched. One more torpedo was fired into the ship to deliver the coup de grace, and about half an hour later Abosso sank, U575 then departing to rendezvous for refuelling.
The one lifeboat which survived was picked up on 31 October by HMS Bideford, one of a convoy forming part of 'Operation Torch', heading for North Africa but, in the heavy seas at the time, no other lifeboats or survivors were ever found.
On the evening of 29 October this year, exactly 80 years later, and as close to the location of the Abosso’s sinking as I could estimate, I sneaked a centrepiece flower arrangement from our table after dinner and, against Cunard’s strictest rules, cast them into the sea at the stern of the ship.
A timekeeper had shown Abosso where the danger was, and the other timekeeper should have saved them. It is a sad thought that if only Captain Tate had left his zig-zag clock running just a little longer, the U-boat would have had to leave and 362 lives would not have been lost.
R.I.P. Uncle Pat.
High-frequency quartz watch fails to resonate
This post was written by James Nye
After the Second World War, Smiths pioneered rebuilding a watch manufacturing industry in Britain. It partnered with Ingersoll to make inexpensive watches in Wales, and developed high-grade watch manufacture at Cheltenham.
Despite achieving strong brand recognition, the economics never worked. Smiths lost money on every watch made at Cheltenham, subsidised from elsewhere in the business. Foreign exchange, material shortages, foreign dumping and more offered overwhelming challenges.
Into 1970 and the head of the clocks and watches division announced a plan to use Swiss watch movements, but in September 1970, Smiths’s CEO announced watch production would cease at Cheltenham, achieved by March 1971.
However, a small team under Dr Shelley of the Special Horological Products Unit continued the development of a cutting-edge quartz watch – the Quasar – launched at the Basle Fair in 1973.

A few hundred pre-production watches were produced. The senior Smiths staff tested them, and when I interviewed some of them ten years ago, a few were recovered from bottom drawers.
One of them was donated and is now in the Clockmakers' Company collection. Through similar generosity, the Clockmakers received a second watch, and also ephemera, including a rough card mounted with parts of the Quasar assembly.

Smiths used an AT-cut quartz crystal, not the normal XY cut. It resonated at 1.5 MHz, against the standard 32,768 Hz, and Smiths actually made this in-house, targeting high performance.
But this came at a price, in size, leaving little room for frequency division. Indeed, ‘conquering the division problem’ was the main achievement remembered years later by former senior Smiths managers.

Omega made a success of their 2.4 MHz AT-crystal watch, but bar or tuning-fork crystals of much lower frequency would soon dominate the mass market in watches by Timex and Junghans. Importantly, Seiko’s 38 Series watches had a frequency of 16,384, achieving better accuracy than the Quasar, with a frequency one hundred times lower!

Volume production of the Quasar never started – Smiths had stacked the odds against itself.
There were problems with the US supply of circuit boards, the stepper motors were hand-wound in Cheltenham, and in-house manufacture of the crystal was both expensive and inefficient.
Pricing was to be pitched at £70 in late 1973, but while watches were still being tested on the wrists of senior management, the chairman and MD of Smiths visited Seiko’s factories, and what they saw convinced them to pull the plug on the Quasar.

Until fairly recently, Quasars had remained essentially invisible – almost mythical. But at a remote auction, a Cheltenham watchmaker's effects turned up. Even if Smiths stopped work in 1973 or so, at least one person kept testing the pre-production samples.
Tags record watch 485 with crystal 419, and watch 365 with crystal 365, faults being found, and adjustments being made.
While the fossil record retains little evidence of a watch that never made it to market, some evidence of its development has nevertheless been preserved.

Clocks in Lisbon
This post was written by Peter de Clercq
Any horologist visiting Lisbon will want to visit the Casa-Museu Medeiros e Almeida, situated just off the majestic Avenida de Liberdade: https://www.casa-museumedeirosealmeida.pt/
It is the house where the Portuguese businessman António Medeiros e Almeida (1895–1986) lived and which he filled with his private collection of objects of decorative arts, including a collection of clocks and watches. It opened as a museum in 2001.
As explained in the catalogue, which I reviewed in Antiquarian Horology in December 2019, it 'consists of a core of about 650 items. [...] The timepieces cover a period of five centuries and are from France, England, Switzerland, Germany, Austria, Italy, Portugal, Russia, China and Japan'. A special gallery was created for them, but clocks are also found in many other rooms.

However, I want to direct you to two other clocks elsewhere in the city.
The first is in the Military Museum, a distinctly old-fashioned – but to me rather inspiring – place; some photos can be found on http://www.exercito.pt/pt/quem-somos/organizacao/ceme/vceme/dhcm/lisboa. On display in one of its enormous rooms is a turret clock with a plaque explaining (I translate from the Portuguese):
'Constructed in Lisbon in the 18th century by the clockmaker F. Baerlein, it was the only turret clock in the neighbourhoods of Santa Apolonia, Santa Clara, Alfama, and surroundings. It commanded all the clocks in the various establishments of the Royal Armoury of the Army, and also serving the population of the nearby areas as well as the crews on the ships on the river Tagus. After being struck by a grenade in May 1915, it stopped functioning. It was repaired in March 1957 […], which the staff of the Military Museum have marked with this plaque.'


A web search found nothing on the maker. What I did find was a photo of the base of a lamp post in Lisbon with the name F. BAERLEIN. Clearly later than the clock movement, but then, perhaps Baerlein had successors in the city?

For the second clock, one must enter the beautiful British Cemetery https://www.britishcemeterylisbon.com/ located opposite the Estrela Park. Tourist guides invariably single out the memorial to the author Henry Fielding (1707–1754), best known for his novel Tom Jones. But to me of far greater interest is the memorial erected for the German-born Charles Louis Christian Rümker (1788–1862) https://www.britishcemeterylisbon.com/interesting-monuments which was the subject of an article by Julian Holland in the Bulletin of the Scientific Instrument Society No. 77 (2003), pp. 32-35.

Rümker served as colonial astronomer in Australia and later as superintendent of the Hamburg Observatory and Nautical School, and the monument shows the tools of the astronomer, including a clock.


While funerary monuments often show an hour glass, symbol of the passing of time, the representation of a clock is certainly rare, perhaps even unique? I would be interested if anyone knows other examples.

The humpback carriage clock
This post was written by William Oke
I have just finished studying on the Horology undergraduate course at Birmingham City University. For my final project, I built a humpback carriage clock and this project allowed me to research surviving examples of these to inform the design, gathering as much information as I could to create a record of those clocks. These are some of my findings.

Abraham Louis Breguet and his son, Antoine-Louis, produced many fine travel clocks including the silver-cased ‘pendule portiques’ that were sold between 1812 and 1830 [1]. Features that are included in nearly all humpback carriage clocks can be traced back to these, including silver engine-turned dials, Breguet’s iconic hand style and a number of complications necessary for travelling such as alarm, calendar work, and moonphase for travelling at night. I have been able to find records of eight of these in various collections.

There are also contemporary examples by the brothers James Ferguson Cole and Thomas Cole. During his life, Cole was called the 'second Breguet' [2]; he made several complex humpback carriage clocks with retrograde astronomical work; the earliest example is from the year of Abraham Louis Breguet's death, 1823 [3]. There is a detailed description of this clock in the first volume of the Horological Journal from the time, which also states seven more were made in the subsequent 30 years [4].

The English tradition of producing these clocks was continued by the London clockmaking firm Jump. The earliest example I have been able to find was sold by Ben Wright, hallmaked 1878 [5]. This clock was made for Francis Baring, 1st Baron Revelstoke, and three further humpback clocks were ordered from Jump by the Baring Bank partners Barons Northbrook, Ashburton and Cromer.
Until this Revelstoke example appeared on the market, the only other of these four whose whereabouts was known was the one made for Ashburton, which came up for sale at Sothebys in 1998. Interestingly, the Ashburton clock was described in a letter to Colonel R. Quill from A. Hayton Jump (great-grandson of the company's founder, R. Jump), who designed 'the last dozen' of these Jump humpback carriage clocks:
“the first of these clocks was made for a Lord Ashburton of the Victorian period before I was in the business. It cost the firm a load of money in time and trouble, for so many men had to make so many parts (the man that made the hands couldn’t do anything else, etc. etc.). My father presented the bill to Lord A with trembling hands and apologised for the high charge. Lord A took the bill and wrote out a cheque at once for double the amount charged on the bill!!! And expressed his appreciation.” [6].
The most recent examples of humpback carriage clocks were produced in the mid-20th century, firstly by Philip Thornton who made several in the immediate postwar years including one that was commissioned by Charles Frodsham & Co. to be presented to Queen Elizabeth, The Queen Mother (who was also in possession of the Breguet pendule portique, No. 2806) [7]. It is likely the last humpback carriage clock was made by Quill and J.S Godman in 1963, which was based on the clocks by Thornton.
These historic examples are rare but have been seen to come up for sale fairly regularly; there is no doubt that their pleasing design and the fine craft skill they display leave them as popular today as when they were first made.
Footnotes:
- Daniels, G., 1975. The Art of Breguet. 2021 ed. London: Philip Wilson Publishers, pp. 78-79.
- The British Horological Institute, 1859. Horological Journal, 1, p. 112.
- Allix, C. & Bonnert, P., 1974. Carriage Clocks, Their History and Development. Woodbridge: Antique Collectors' Club Ltd.
- The British Horological Institute, 1859. Horological Journal, 1, pp. 134-135.
- Ben Wright Clocks Ltd, 2016. Jump, London. https://www.benwrightclocks.co.uk/clock.php?i=105 [Accessed 3 May 2021].
- Allix, C. & Bonnert, P., 1974. Carriage Clocks, Their History and Development. Woodbridge: Antique Collectors' Club Ltd, pp. 289-90.
- The Royal Collection Trust, n.d. Carriage clock 1947. https://www.rct.uk/collection/2943/carriage-clock [Accessed 18 April 2022].
The creation of 'A General History of Horology'
This post was written by James Nye
Winston Churchill wryly commented, ‘Writing a book is an adventure. To begin with it is a toy and an amusement. Then it becomes a mistress, then it becomes a master, then it becomes a tyrant. The last phase is that just as you are about to be reconciled to your servitude, you kill the monster and fling him to the public.’

The emergence of Oxford University Press’s General History of Horology has echoed some of these observations. The editors do not necessarily agree, but I believe it was first in a bar in Pasadena, during the 2013 NAWCC conference on ‘Time for Everyone’, that Anthony Turner first broached the idea of a major work on the history of horology. I think everyone smiled politely.
Years later, in October 2016, he asked me if I would help, as his ideas were formulating and he had already managed to get Jonathan Betts interested in collaborating. However, both of us told Anthony that we were heavily committed, and I can see our emails were more than a bit tentative.

In June 2018, Anthony was back in touch, as he had now convinced OUP to get on board with the project. We met at a London hotel in July that year, and over a convivial and euphoric dinner Betts and I agreed to get fully on board as advisory editors and a firm proposal went to OUP.
August 2018 saw the beginnings of establishing the wider team of authors. The book ended up with thirty-five writers, though originally there were more.

In November 2018, OUP came back with comments from the external referees who had examined the proposal. One of them was hoist with his own petard when Anthony insisted that the referee himself fill a gap that he had indicated in the work.
A manuscript delivery deadline was set at December 2019. A draft contract emerged in January 2019, between OUP, the editors, and each contributor, eventually signed off in August 2019.
By May 2019, authors were beginning to discuss chapters with each other. It was at this point – when the euphoria had faded – that I recall joint authors were beginning to admit to each other they had bitten off what seemed like more than they could chew.
Helpfully, there were positive discussions with Sotheby’s on sponsorship. This allowed us to have colour illustrations throughout and a professionally compiled index.
Over the following months, draft chapters were beginning to fly around. Some of these were written in other languages, and needed translating to English. And more than a few were penned by Anthony himself, of course.
We had eight chapters in all by September 2019, but at this point we were also losing authors, who realised that other commitments stood in their way. And other authors were commenting, ‘Yes, I really must get down to some work!’
Unfortunately, one scholar from whom we had hoped to have a lengthy contribution died even before we could invite him. Health problems held up contributions from others.
The submission deadline shifted to late February 2020 to give us a chance to edit the whole work and to submit in turn to OUP in October that year. In January 2020 we were still scrabbling to find authors to fill a few missing parts of the jigsaw, and the timetable was under pressure. Chapters kept arriving, again some needing translation by Anthony, and some drastic editing as at least two were twice as long as required.
Eighteen chapters were in by mid-February, but as a contributing author as well as an editor, I was beginning to feel a bit of pressure myself!

The fateful month of March 2020 arrived, bringing Covid. More material arrived, but another author was lost owing to external commitments, necessitating further re-arrangement. By May we had to increase discipline significantly, since we had so many chapters on board, and three editors considering them – version control was vital.
If there had been excuses before, lockdown removed them all, and towards the end of the year the last of our contributions were coming together. It was early November 2020 that we finally had the whole book in draft, and much of that month was taken up in sorting images and securing consents.
We began considering a cover in early 2021 and realised that it was necessary to look beyond accurate representations of any clocks or watches. Thus, the idea of commissioning a bespoke artwork from Lee Yuen-Rapati emerged.

In February 2021, production work on the book began in earnest. We saw first proofs at the beginning of July, and the second half of the year was taken up with gradually ensuring that myriad small corrections were made.
In early December, we realised that there were major problems with the index that had been provided, and the work had to be restarted. Tragically, Paulo Brenni, one of our authors, unexpectedly died.
We finally had a complete and workable index by mid-January 2022, with OUP sending all the files to the printers in February, while the paper actually ran through the presses on 28 March. It was then that the physical production of a slipcase managed to sabotage the timetable, and books only finally started arriving in late June.
It was quite a ride along the way. I see my computer has 27GB of material relating to the project, and at least 1,300 emails. Anthony’s statistics will be larger, given his role as General Editor.
Our aim was to offer a truly international treatment of the subject. In production it was also international. The book was written in nine countries, edited in England and France, designed and typeset in India, indexed in England and printed in Scotland.
But at least this global monster has been killed – we trust the public finds the struggle worth it.
You can order the book here. Use the promo code ASPROMP8 to save 30 per cent.
The two videos of the book being produced are courtesy of Bell & Bain Ltd, Glasgow.